Smelting is a form of extractive metallurgy; its main use is to produce a metal from its ore. This includes iron extraction from iron ore, and copper extraction and other base metals from their ores. Smelting uses heat and a chemical reducing agent to change the oxidation state of the metal ore; the reducing agent is commonly a source of carbon such as coke, or in earlier times charcoal. The carbon or carbon monoxide derived from its removes oxygen from the orde to leave the metal. The carbon is thus oxidized in two stages, producing first carbon monoxide and the carbon dioxide. As most ores are impure, it is often necessary to use flux, such as limestone, to remove the accompanying rock gangue as slag. Smelting involves more than just "melting the metal out of its ore. To produce the metal, these compounds have to undergo a chemical reaction. Smelting therefore consists of using suitable reducing substances that will combine with those oxidizing elements to free the metal.
In nature, the ores of metals such as iron, copper, lead, aluminum and othe metals are found impure states, often oxidized and mixed in with silicates of other metals. During smelting when the ore is exposed to high temperatures, these impurities are seperated from the molten metal and can be removed. The collection of compounds that is removed is the Slag. In many smelting processes, oxides are introduced to control the slag chemistry, assisting in the removal of impurities and protecting the furnace refactory lining frome excessive wear.

Basic slag is a byproduct of steelmaking by the basic version of the bessemer process or the linz-donawitz process. It is largely limestone or dolomite which has absorbed phosphate from the iron ore being smelted. Because of the slowly released phostphate content, as well as for its liming effect, it is valued as fertilizer in gardens and farms in steelmaking areas
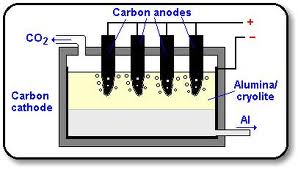
Aluminum production from bauxite ore is a three step process. First the alumina is extracted from bauxite ore usually using the Bayer Process. In the Bayer Process, finely crushed bauxite is mixed with sodium hydroxide and placed in a `digester.' High temperatures and pressures in the digester cause reactions in the ore / sodium hydroxide mixture. The result is dissolved aluminum oxide and ore residue. The residues, which include silicon, lead, titanium, and calcium oxides, form a sludge in the bottom of the digester. The aluminum oxide is evaporated off and condensed. Starches and other ingredients are added to remove any remaining impurities from the oxide.
The solution is then moved to a precipitation tank where the aluminum oxide is crystallized. Aluminum hydroxide and sodium hydrizide are the products of the crystallization. The crystals are washed, vacuum dewatered and sent to a calcinator for further dewatering.
.
Roasting
In the case of carbonates and sulfides, a process called roasting drives off the unwanted carbon or sulfur, leaving an oxide, which can be directly reduced. Roasting is usually carried out in an oxidizing environment.
Reduction
Reduction is the final, high temperature step in smelting. It is here that the oxide becomes the elemental metal. A reducing environment, pulls the final oxygen atoms from the raw metal. The required temperature varies over a very large range, both in absolute terms, and in terms of the melting point of the base metal.
Fluxes
Fluxes are used in smelting for several purposes, chief among them catalyzing the desired reactions and chemically binding to unwanted impurities or reaction products. Calcium oxide, in the form of lime, was often used for this purpose, since it could react with the carbone dioxide or sulfur dioxide produced during roasting and smelting to keep them out of the working environment.