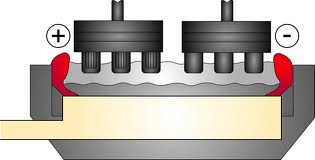
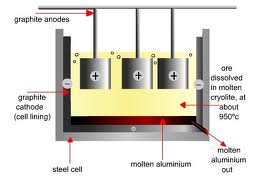
The Hall Heroult Proces is the major industrial process for the production of aluminum. In the Hall Heroult Process alumina, is dissolved in a carbon-lined bath of molten cryolite. Aluminum flouride, is also present to reduce the melting point of the cryolite. The mixture is electrolized, and liquid aluminum is produced at the cathode. The carbon anode is oxidized and bubbles away as carbon dioxide.
The liquid aluminum product is denser than the molten cryolite and sinks to the bottom of the bath where is it periodically collected. The top and sides of the bath are covered with a crust of solid cryolite which acts as thermal insulation electrical resistance within the bath provides sufficient heat to keep the cryolite molten.

The Hall Heroult Process is used all over the world, and is the only method of aluminum smelting currently used in the industry. Today there are two primary technologies using the hall heroult process: Soderberg and Prebake. Soderberg uses a continuously created anode made by addition of "pitch" to the top of the anode. The lost heat from the smelting operation is used to bake the pitch into the carbon form required for reaction with alumina. Prebake technology is named adter its anodes, which are baked in very large gas-fired ovens at high temperature before being lowered by various heavy industrial lifting systems into the electrolytiic solution. In both technologies, the anode, attached to a very large electrical bus, is slowly used by the proceess. Prebake technology tends to be slightly more efficient, but is more labor intesive. In both technologies the anode, attached to a very large electrical bus is slowly used up by the process. Prebake tends to be slightly more efficient, but is more labor intensive. Prebake is becomming preferred in the industry because of the various pollutant emissions related to the creation of the anode from liquid pitch.
Aluminum Primary smelting and casting
Primary aluminum is produced in reduction plants where pure aluminum is extracted from alumina by the hall heroult process. The reduction of alumina into liquid aluminum is operated at around 950 degrees celsius. This process takes place in electrolytic cells, where carbon cothodes from the bottom of the pot and act as the negative electrode. Anodes are help at the top of the pot and are consumed during the process when they react with the oxygen coming from the alumina. There are two types of anodes currently in use. All pot lines built since the early 1970s use the prebake anode technology, where the anodes, manufactured from a mixture of petroleum coke and coal tar pitch, are pre-baked in seperate anode plants. In the soederberg technology, the carbonceous mixture is fed directly into the top part of the pot, where self bakin anodes are produced using the heat released by the elctrolytic process.